

When temperature change : Use mc@
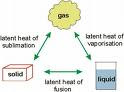
When there is change in state: Use mL
To avoid confusion, draw a diagram in the form of a thermometer.
Mark out the higher temperature and the lower temperature and the massess involved. Mark out the common temperature. The temperature change for the heat released and heat absorbed can be determined easily (ie no need final -initial temp)
Equate the heat released to heat absorbed. Substitute the terms correctly paying special attention to changes in state. Remember water is 4200 Jkg*-1K*-1. Ice and Steam have different specific heat capacities. Ice and steam also have different latent heat.
Prepare thermal 2, you only need to be careful to succeed in getting the right answer.